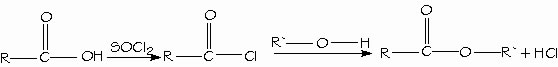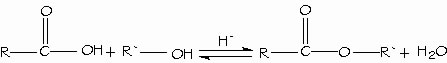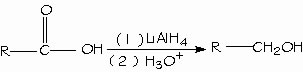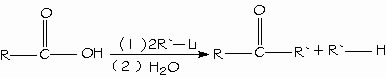Carboxylic acids
Summary
The carboxyl group is one of the most abundant functional groups in chemistry and biochemistry. Not only are the carboxylic acids themselves important, but the carboxyl group is the group from which a large family of compounds is derived.
It is a primary carbon function. It is characterized by having on the same carbon the carbonyl group and an oxhydryl. They are named by prefixing the word acid with the suffix oic. Some of them are better known by their common names such as formic acid (methanoic) and acetic acid (ethanoic).
Keywords: carboxyl, chemistry, carbon, acid.
Abstract
The carboxyl group is one of the most abundant functional groups in chemistry and biochemistry. Not only are carboxylic acids important in themselves, but the carboxyl group is the group from which a large family of compounds is derived. It is a primary carbon function. It is characterized by having at the same carbon the carbonyl group and an oxyhydryl. They are named before the acid word and with the oico suffix. Some of them are better known by their common names like formic acid (methanoic) and acetic acid (ethanoic).
Keywords: Carboxyl, chemical, carbon, acid.
Definition of carboxylic acid
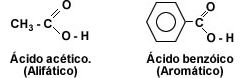
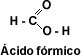
Carboxylic acids are compounds characterized by the presence of a carboxyl group (-COOH) attached to an alkyl or aryl group. When the carbon chain has only one carboxyl group, the acids are called monocarboxylic or fatty acids, so called because they are obtained by hydrolysis of fats.
The first member of the aliphatic series of carboxylic acids is methanoic acid or formic acid, this acid is found in nature secreted by ants when biting.
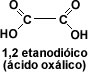
The first member of the aromatic group is phenylmethanoic or benzoic acid. When the carbon chain has two carboxyl groups, the acids are called dicarboxylic acids, the first member of the aliphatic series being 1, 2-ethanedioic or oxalic acid.
Nomenclature
Carboxylic acids are named with the help of the ending -oic or -ico which is joined to the name of the reference hydrocarbon and prefixed with the word acid:
Example
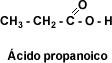
CH3-CH2-CH3 propane CH3-CH2-COOH Propanoic acid (propan + oic)
In the IUPAC system, the names of the carboxylic acids are formed by replacing the "o" ending of the alkanes by "oic", and prefixing the word acid.
The skeleton of alkanoic acids is numbered by assigning No. 1 to the carboxylic carbon and continuing with the longest chain that includes the COOH group.
In the carboxyl functional group, a hydroxyl (-OH) and carbonyl (-C=O) group coincide on the same carbon. It can be represented as -COOH or -CO2H.
Physical properties
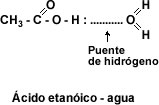
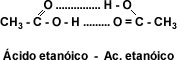
Solubility: The carboxyl group -COOH confers polar character to acids and allows the formation of hydrogen bridges between the carboxylic acid molecule and the water molecule. The presence of two oxygen atoms in the carboxyl group makes it possible for two acid molecules to bond together by double hydrogen bridging, forming a cyclic dimer.
image ethanoic acid.jpg]"This makes the first four aliphatic monocarboxylic acids completely water-soluble liquids. Solubility decreases as the number of carbon atoms increases. From dodecanoic acid or lauric acid onwards the carboxylic acids are soft solids insoluble in water.
In monocarboxylic aromatic acids, the carbon to carbon ratio is 6:1 which causes the solubility to be decreased with respect to aliphatic monocarboxylic acids.
Carboxylic acids are soluble in less polar solvents, such as ether, alcohol, benzene, etc. Carboxylic acids boil at even higher temperatures than alcohols. Such high boiling points are due to the fact that a pair of carboxylic acid molecules are held together not by one but by two hydrogen bonds. The odors of the lower aliphatic acids progress from the strong and irritating formic and acetic to the overtly unpleasant butyric, valeric and caproic acids; the higher acids have very little odor because of their low volatilities.
Boiling point: Carboxylic acids exhibit high boiling points due to the presence of double hydrogen bridging.
Melting point: The melting point varies according to the number of carbons, being higher for formic and acetic acids, when compared to propionic, butyric and valeric acids of 3, 4 and 5 carbons, respectively. After 6 carbons the melting point rises unevenly.
This is because the increased number of carbon atoms interferes with the association between the molecules. Aromatic monocarboxylic acids are crystalline solids with higher melting points than aliphatic acids.
Formic and acetic acids (1, 2 carbons) are irritating smelling liquids. Butyric, valeric and caproic acids (4, 5 and 6 carbons) have unpleasant odors. Acids with a higher number of carbons have a low odor.
The salts of carboxylic acids are non-volatile crystalline solids consisting of positive and negative ions and their properties are those corresponding to such structures. The considerable electrostatic forces holding the ions in the crystalline lattice can only be overcome by heating at elevated temperature or by means of a very polar solvent. The temperature required is so high that, before it is reached, carbon-carbon bonds are broken and the molecule decomposes, which generally occurs between 300-400°C. A decomposition point is rarely useful for the identification of a substance, since it usually reflects the rapidity of heating rather than the identity of the compound.
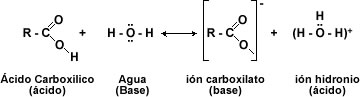
A strong base can completely deprotonate into a carboxylic acid. The products are the carboxylate ion, the remaining cation of the base, and water. The combination of a carboxylate ion and a cation constitutes the salt of a carboxylic acid.
Chemical properties
The chemical behavior of carboxylic acids is determined by the carboxyl group -COOH. This function consists of a carbonyl group (C=O) and a hydroxyl group (-OH). Where the -OH is the one that undergoes almost all reactions: loss of proton (H+) or replacement of the -OH group by another group.
Formation of nitrile hydrolysis. The best reagents for converting carboxylic acids to acid chlorides are thionyl chloride (SOCl2) and oxalyl chloride (COCl)2, because they form gaseous by-products that do not contaminate the product. Oxalyl chloride is very easy to use because it boils at 62°C and evaporates from the reaction mixture.
Synthesis and use of acid chlorides. Carboxylic acids are directly converted to esters by Fischer esterification by reacting with an alcohol with acid catalysis.
Condensation of acids with alcohols. Fischer esterification. Lithium aluminum hydride (LiAlH4) reduces carboxylic acids to form primary alcohols. The aldehyde is an intermediate in this reaction, but cannot be isolated because it is more easily reduced than the original acid.
Reduction of carboxylic acids. A general method for preparing ketones is the reaction of a carboxylic acid with 2 equivalents of an organolithium reagent.
Alkylation of carboxylic acids to form ketones
Decarboxylation of carboxylate radicals
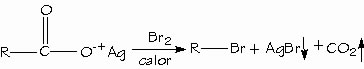
A chemical reaction in which a carboxyl group is removed from a compound in the form of carbon dioxide (CO2).
Carboxylic acids can be converted to alkyl halides with loss of a carbon atom by the Hunsdiecker reaction.
Utility in the carboxylic acid industry
The carboxylic acids of greatest industrial application are acetic acid, which is mainly used to obtain vinyl acetate, which is used as a monomer for the manufacture of polymers.
It is also used in the production of cellulose acetate to obtain lacquers and photographic films, as well as in the manufacture of solvents for resins and lacquers.
The aluminum salt of acetic acid is used as a mordant in dyeing. Formic acid is often used in the tanning industry to soften hides and skins and also in dyeing processes in the tanning industry. Some chlorinated derivatives of carboxylic acids are used in the production of herbicides.
Benzoic acid is widely used as a synthesis intermediate in many organic processes and some of its esters are used as plasticizers and in the perfume industry (benzyl benzoate). Sodium benzoate is used in the food industry as a preservative (juices, soft drinks, jams, etc.).
Among the dicarboxylic acids, propanedioic acid (malonic acid) is used in the production of medicines, pesticides and dyes. 1-4-butanedioic acid (succinic acid) is used in the production of polyester resins for varnishes and trans-butenodioic acid (fumaric acid) is used as an acidulant in the manufacture of soft drinks.
Bibliography
McMurry J. Organic Chemistry. Mexico; DF. 6th ed. Thomson 2004
Vollhardt, K. Peter. Organic Chemistry. 3rd edition. Omega. Madrid. 2000. pp. 849-850
Ray Q. Bruwster. Organic Chemistry. Compañía editorial continental, S.A. Mexico. D.F.1970.
Solomons, T.W. Graham e María Cristina Sangines Franchini Química orgánica. Mexico, D.F.: Limusa, 1985.
Petrucci, GENERAL CHEMISTRY. Pearson, eighth edition, Spain, Madrid 2003.
Guillen, Gimeno.1999. CHEMISTRY, Ed. Laberinto, Spain.
http://www.salonhogar.net/quimica/nomenclatura_quimica/Propiedades_acidos_carboxilicos.htm
[a] Teacher Escuela Preparatoria No. 3
Share in: